The newest line of investigation in my laboratory is centered on evaluating the participation of cells of the innate immune system in the pathogenic sequence of events that occur in the testis after exposure to the Sertoli cell toxicant mono-(2-ethylyhexyl) phthalate (MEHP). Phthalates are a class of compounds that are incorporated into cosmetics, food packaging, biomedical devices, and PVC products to impart flexibility. Since these agents are not covalently bound to the final product they readily leach out of these products and, as a result, are found widely distributed in the environment and human tissues. The most abundantly produced and studied phthalate is di-(2-ethylhexyl) phthalate (DEHP) that is rapidly hydrolyzed in the gastrointestinal tract, liver and blood, by nonspecific esterases to produce its corresponding toxic monoester, MEHP. Although the collective human median phthalate exposure level is estimated to be ~30 µg/kg/bw/day, certain individual populations, such as young children receiving parenteral fluids via polyvinyl-chloride based tubing, show levels of 10-20 mg/kg/day. It is widely recognized that peripubertal rodents (~postnatal day, PND, 14-28) are more sensitive than adults to testicular injury by phthalates. The work of this research project is anticipated to provide key insights into the mechanisms that account for the age-dependent susceptibility to phthalate-induced testicular injury.
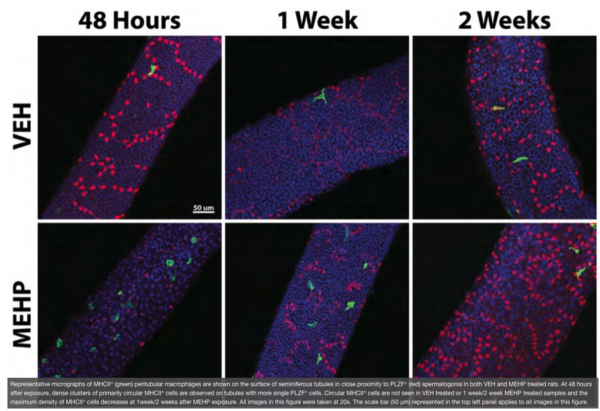
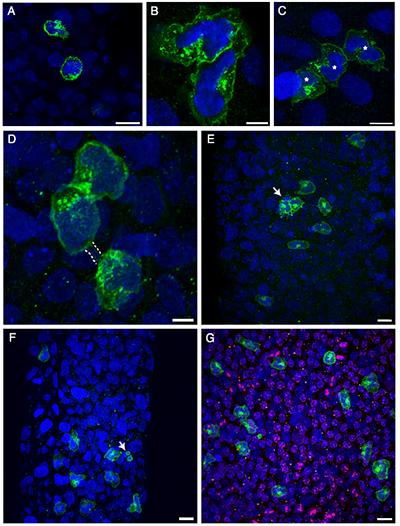
The testis is referred to as an “immune privileged” organ as it protects auto-antigenic haploid GCs that appear at puberty, long after the establishment of immune tolerance mechanisms. The immune privilege mechanism is maintained by a specialized physical barrier that exists between adjacent SCs, the blood-testis barrier, and the secretion of immunosuppressive molecules from cells of the seminiferous epithelium, predominantly by Sertoli cells. Therefore, exposure to MEHP may create a disruption in the testis’ immunosuppressive environment and allows for the infiltration of these cells into the testis in numbers not normally observed. In agreement with this idea, we have reported a MEHP dose dependent infiltration of macrophages and neutrophils into the testis that occurs in a time-, age- (postnatal day vs adult age), and species- (Fischer rats vsC57BL/6J mice) dependent manner. Nevertheless, experimental approaches that significantly reduced the levels of macrophage infiltration into the testis failed to prevent MEHP-induced increases GC apoptosis. Interestingly, our recent detailed evaluation of the cells that lie adjacent to the basement membrane of the seminiferous tubules revealed a specific sub-population of macrophages, the peritubular macrophages (ptMφs), that are increased in numbers as well as in specific regional clusters for a sustained period (>2 weeks) after MEHP exposure. The ptMφs in the testis are thought to play a critical role in the maintenance of the microenvironment supporting the spermatogonial niche. In this research project we challenge the hypothesis that the ptMφs cells arise from a phenotypic switch from the infiltrating macrophages that occur in response to MEHP exposure and that their function is to facilitate the efficient recovery of spermatogenesis.
Relevant Manuscripts:
Gillette, R, Tiwary, R, Voss, JJLP, Hewage, S and Richburg, JH (2021). Peritubular macrophages are recruited to the testis of peri-pubertal rats after mono-(2-ethylhexyl) phthalate exposure and is associated with increases in the numbers of spermatogonia. Toxicological Sciences. 182 (2), 288-296. PMC8331146. ***Awarded "Best Paper" of 2021 in the journal Toxicological Sciences by the Society of Toxicology's Reproductive and Developmental Toxicology Specialty Section.
Gillette, R, Tiwary, R, Voss, JJLP and Richburg, JH (2019). Peritubular macrophages and spermatogonia are sequentially increased in the testis of rats after mono-(2-ethylhexyl) phthalate exposure. BioRXiv. Posted online Sep. 12, 2019; doi: 10.1101/767707
Voss, JJLP, Stermer, AR, Ghaffari, R, Tiwary, R, and Richburg, JH (2018). MEHP-induced rat testicular inflammation does not exacerbate germ cell apoptosis. Reproduction. 156 (1): 35-46. PMCID: PMC6021206
Stermer, AR, Murphy, CJ, Ghaffari, R., Di Bona, KR, Voss, J.J. and Richburg, JH (2017). Mono (2-ethylhexyl) phthalate-induced Sertoli cell injury stimulates the production of pro-inflammatory cytokines in Fisher 344 rats. Reproductive Toxicology. 69: 150-158. PMCID: PMC5406244.
Stermer, AR, Myers, JL, Murphy, CJ, Di Bona, KR and Richburg, JH (2016). Female mice with a loss-of-function ITCH display an altered reproductive phenotype. Experimental Biology and Medicine. 241 (4):367-374. Epub 2015 Oct 28. PMCID: PMC4935413 **This was the featured article for the February 2016 Issue**.
Murphy, CJ, Stermer, AR and Richburg, JH (2014). Age- and species-dependent infiltration of macrophages into the testis of rats and mice exposed to mono-(2-ethylyhexyl) phthalate (MEHP). Biology of Reproduction. 91(1):18, 1-11. PMCID: PMC4434960
Project 2: Deciphering mechanisms of cisplatin-induced male infertility
Project Overview:
In 1972, the introduction of cis-diamminedichloroplatinum (II) (cDDP, cisplatin) for the treatment of testicular germ cell tumors dramatically increased the overall cure rate and patient survivorship. Unfortunately, since testicular cancers occur most commonly in young men ~15-34 years of age, the prolonged, sometimes permanent, infertility that often results from cDDP treatment is a devastating side effect for these young men. We (Seaman et al., 2003 Apoptosis 8 (1):101-108), and others have shown that testicular germ cells are extremely sensitive to undergo apoptosis after cDDP exposure. The germ cell subtypes reported to undergo cDDP-induced cell death in rodent models include undifferentiated and differentiated spermatogonia as well as spermatocytes; with subtype sensitivity closely dependent on both the dose and developmental age of the animal. We developed a cDDP multi-cycle dosing paradigm in mice that closely mimics that used in the clinic and showed that this treatment results in the prolonged disruption in spermatogenesis in these mice (Sawhney et al., 2005 Journal of Andrology 26:136-145) and that the undifferentiated spermatogonia were increasingly affected by multiple cycles of cDDP treatment (Harman et al., 2014 Toxicology Letters 227:99-112). Although spermatogonial stem cells (SSCs) are regarded to be mostly insensitive to DNA-damaging agents like cDDP due to their quiescent nature, it has recently been shown in the lab of Shosei Yoshida (2014, Cell Stem Cell14:658-672) that the progeny of SSCs, the undifferentiated spermatogonia, can dedifferentiate into SSCs and serve as a mechanism for maintaining the functional stem cell pool throughout the life of the animal. Therefore, the prolonged loss of spermatogonia that we have observed in mice using the cDDP multi-cycle dosing paradigm in mice may negatively affect the size of the testicular SSC pool, reducing male fertility. This research project is specifically targeted to test if cDDP is preferentially taken up in specific germ cell subtypes via their enhanced expression of the high affinity membrane copper transporter 1 (CTR1; SLC31A1) and accounts for their distinctive sensitivity to elimination after cDDP exposure. This hypothesis is based upon the growing body of evidence in the literature indicating that CTR1 is the major influx transporter for cDDP. As well as our preliminary observations showing a robust expression of this protein in the testis and that the testis of Ctr1+/- mice show a limited loss of germ cells after 2 treatment cycles of cDDP. Although our hypothesis predicts that germ cell CTR1 underlies the sensitivity of these cells to cDDP-induced apoptosis, the evaluation of CTR1 in other cells of the seminiferous epithelium (e.g., Sertoli cells) is also closely considered as CTR1-mediated cDDP influx in other testicular cells could also contribute to the pathogenic mechanism responsible for the sustained loss of germ cells after a repeated multi-cycle treatment of cDDP by disrupting the maintenance of the microenvironment necessary for germ cell development.
It is anticipated that this research will impact the field by offering a mechanistic foundation to explain the distinct sensitivity of testicular germ cells to cDDP-induced elimination thus providing insights for the development of translational research into innovative clinical strategies that will continue to allow for the efficient elimination of tumor cells by cDDP while sparing the long-term fertility of men.
Relevant Publications:
Ghaffari, R, Di Bona, KR, Riley, CL and Richburg, JH (2019). Copper transporter 1 (CTR1) expression by mouse testicular germ cells, but not Sertoli cells, is essential for functional spermatogenesis. PlosOne. 14 (4) e0215522
Ghaffari, R, and Richburg, JH (2019). Mice with a Sertoli cell-specific knockout of the Ctr1 gene exhibit a reduced sensitivity to cisplatin-induced testicular germ cell apoptosis. Toxicology Research. DOI: 10.1039/C9TX00142E
Harman, J and Richburg, JH (2014). Cisplatin-induced alterations in the functional stem cell pool and niche in C57BL/6J mice following a clinically relevant multi-cycle exposure. Toxicology Letters Apr 2;227(2):99-112.
Sawhney, P, Giammona, CJ, Meistrich, ML and Richburg, JH (2005). Cisplatin-induced long-term failure of spermatogenesis in adult C57/Bl/6J mice. Journal of Andrology 26:136-145.
Seaman FC, Sawhney, P, Giammona, CJ and Richburg, JH (2003). Cisplatin-induced pulse of germ cell apoptosis precedes long-term elevated apoptotic rates in C57/B6 mouse testis. Apoptosis 8 (1):101-108.