
Professor of Chemical Biology & Medicinal Chemistry
Alan W. Hamm Centennial Fellow in Pharmacy
Laboratory of DNA damage and repair and DNA-targeting drugs
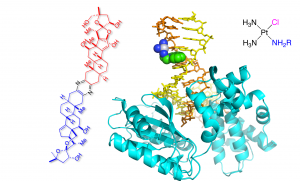
Our research program aims to gain molecular-level insights into the mechanisms of DNA damage, repair, mutagenesis, translesion synthesis, and DNA-targeting anticancer drugs. We are particularly interested in oxidative (e.g., 8-halogenated guanine, 8-oxoguanine) and alkylative (e.g., N7-alkylguanine, intra- and interstrand cross-links) DNA lesions that are formed by a wide variety of mutagens (e.g., reactive oxygen or halogen species), carcinogens (e.g., nitrosamines, ptaquiloside) and chemotherapeutics (e.g., nitrogen mustards, platinum-based drugs). Our research questions include: How do oxidative or alkylative DNA lesions affect DNA structures and base pairing properties? What are the mechanisms by which DNA repair machineries (e.g., base and nucleotide excision DNA repair, interstrand cross-link repair) recognize and process their substrate lesions? How do translesion synthesis DNA polymerases (e.g., polη, polβ) accurate or promutagenic bypass of DNA lesions? How do DNA lesions induce mutagenesis and carcinogenesis? What are the molecular mechanisms by which mono-functional platinum-based chemotherapeutics induce cancer cell death? We utilize combined tools of synthetic chemistry, biochemistry, and structural, cellular and molecular biology to address these questions.
DNA damage, repair, replication, and mutagenesis
Cancer is a genetic disease, which often involves mutations in critical genes such as tumor-suppressor genes and oncogenes. The human genome is persistently attacked by DNA damaging agents, such as reactive oxygen species and alkylating agents, to form various DNA lesions. The vast majority of DNA lesions are removed by DNA repair machinery (e.g., base excision DNA repair, nucleotide excision DNA repair), but a small portion of the lesions evade the repair mechanisms and induce mutations during replication. We are currently evaluating the effects of DNA alkylation, oxidation, halogenation and platination on DNA replication and mutagenesis.
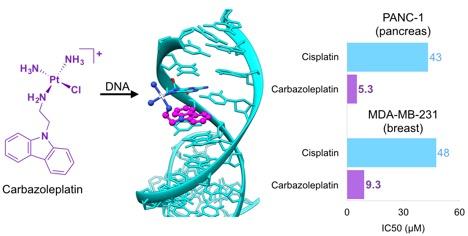
Developing platinum-based chemotherapeutics: Platinum-based agents are among the most widely prescribed chemotherapeutic drugs. Cisplatin and its analogs are used to treat approximately half of all cancer patients receiving chemotherapy. The antitumor activity of cisplatin mainly stems from its ability to form intrastrand cross-link, which deters DNA replication and transcription and initiates a signaling cascade that leads to cell death. The major limitation of the cisplatin-based chemotherapy is its resistance. Our research goal is to develop platinum-based anticancer agents with a new mode of action and a reduced resistance.
48. Genotoxic C8-arylamino-2´-deoxyadenosines act as latent alkylating agents to induce DNA interstrand cross-links. Rozelle AL, Lee S. J. Am. Chem. Soc 2021, https://doi.org/10.1021/jacs.1c07234
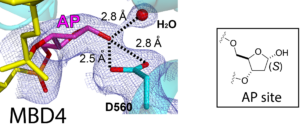
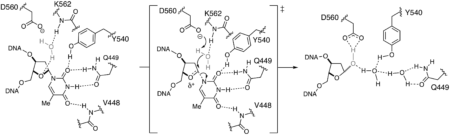
47. Insights into the substrate discrimination mechanism of methyl-CpG-binding domain 4. Ouzon-Shubeita H, Schmaltz LF, Lee S. Biochem. J. 2021, 478, 1985-1997.
46. Insights into the mismatch discrimination mechanism of Y-family DNA polymerase Dpo4. Jung H, Lee S. Biochem. J. 2021, 478, 1769-1781.
45. DNA interstrand cross-links induced by the major oxidative adenine lesion 7,8-dihydro-8-oxoadenine. Rozell AL, Cheun Y, Vilas CK, Koag MC, Lee S. Nature Commun. 2021, 12, 1897.
44. Translesion synthesis of the major nitrogen mustard-induced DNA lesion by human DNA polymerase η. Jung H, Rayala NK, Lee S. Biochem. J. 2020, 477, 4543-4558.
43. Structural insights into the bypass of the major deaminated purines by translesion synthesis DNA polymerase. Jung H, Hawkins M, Lee S. Biochem. J. 2020, 477, 4797-4810.
42. Promutagenic bypass of 7,8-dihydro-8-oxoadenine by translesion synthesis DNA polymerase Dpo4. Jung H, Lee S. Biochem. J. 2020 477, 2859-2871 [PMC7448247].
41. Catalytic mechanism of the mismatch-specific DNA glycosylase methyl-CpG-binding domain 4. Ouzon-Shubeita H, Jung H, Lee MH, Koag MC, Lee S. Biochem J. 2020, 477, 1601-1612.
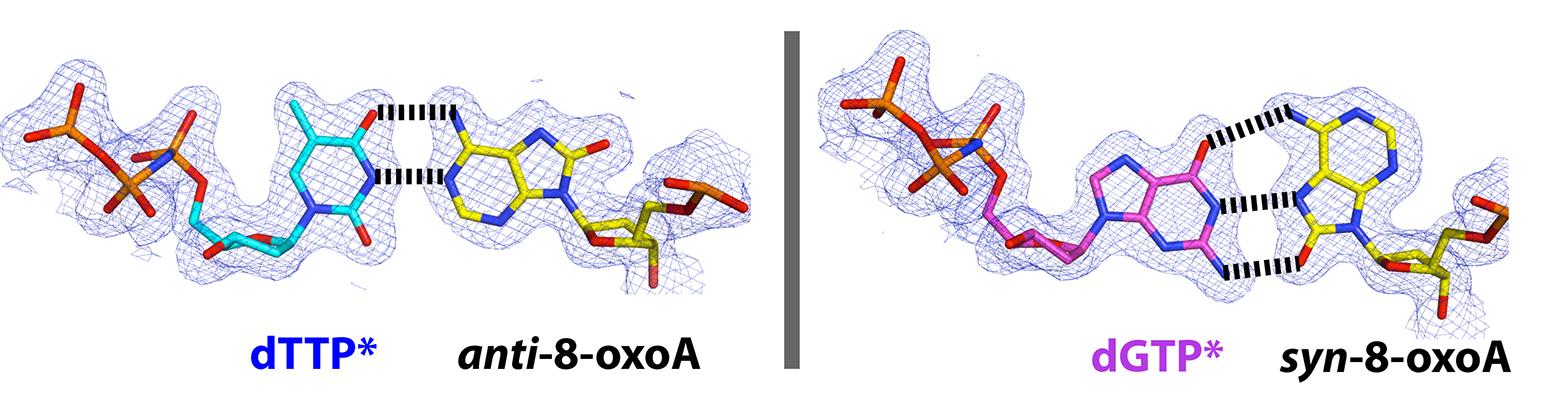
40. Mutagenesis mechanism of the major oxidative adenine lesion 7,8-dihydro-8-oxoadenine. Koag MC, Jung H, Lee S. Nucleic Acids Res. 2020, 48, 5119-5134.
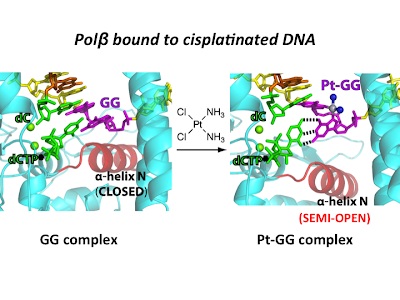
39. Structural insights into the promutagenic bypass of the major cisplatin-induced DNA lesion. Ouzon-Shubeita H, Vilas CK, Lee S. Biochem J. 2020, 477, 937-951.
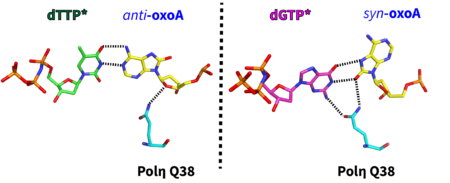
38. Bypass of the major alkylative DNA lesion by human DNA polymerase η. Koag MC, Jung H, Kou Y, Lee S. Molecules 2019, 24, 3928.
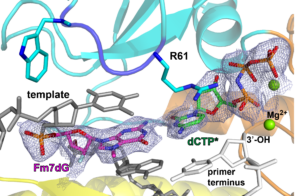
37. Promutagenicity of 8-chloroguanine, a major inflammation-induced halogenated DNA lesion. Kou Y, Koag MC, Lee S. Molecules 2019, 24, 3507.
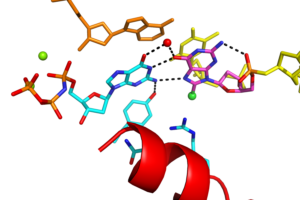
36. Photo-induced DNA interstrand cross-links formed by a coumarin-modified nucleoside. Rozelle AL, Kumar RN, Lee S. Nucleosides Nucleotides Nucleic Acids. 2019;38(3):236-247.
35. Mutagenic replication of the major oxidative adenine lesion 7,8-dihydro-8-oxoadenine by human DNA polymerases. Koag MC, Jung H, Lee S. J Am Chem Soc. 2019, 141(11):4584-4596.
34. Structural basis for the bypass of the major oxaliplatin-DNA adducts by human DNA polymerase η. Ouzon-Shubeita H, Baker M, Koag MC, Lee S. Biochem J. 2019 476(4):747-758. doi: 10.1042/BCJ20180848.
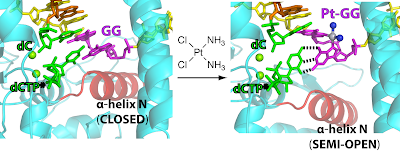
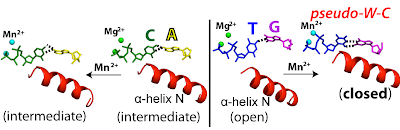
33. Structural and kinetic studies of the effect of guanine N7 alkylation and metal cofactors on DNA replication. Kou Y, Koag MC, Lee S. Biochemistry. 2018, 57, 5105-5116.
32. Insights into the effect of minor groove interactions and metal cofactors on mutagenic replication by human DNA polymerase β. Koag, MC; Lee, S. Biochem J. 2018 Feb 9;475(3):571-585. (recommended for F1000Prime).
31. Synthesis and bioactivity of bis-steroidal pyrazine 23-deoxy-25-epi ritterostatin GN1N. Kumar, RN.; Lee, S.* Steroids 2017, 126, 74-78.
30. Synthesis, structure, and biological evaluation of a platinum-carbazole conjugate. Cheun Y, Koag MC, Naguib YW, Ouzon-Shubeita H, Cui Z, Pakotiprapha D, Lee S. Chem Biol Drug Des. 2017 doi: 10.1111/cbdd.13062.
29. Synthesis of 23-deoxy-25-epi north unit of cephalostatin 1 via reductive and oxidative modifications of hecogenin acetate. Kumar, N.; Lee, S.* Steroids 2017, 118, 68-75
28. cis-Diammine[3-(3-chloro-7-methoxy-9,10-dihydroacridin-9-ylideneamino)propan-1-aminej2 N,N0]platinum(II) dinitrate. Cheun, Y.; Lee, S*. IUCrData (2016). 1, x160481.
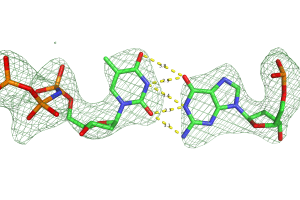
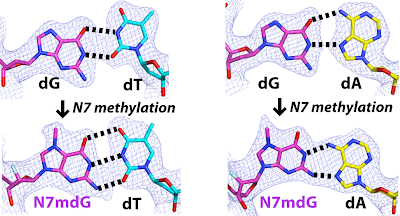
27. N7 methylation alters hydrogen-bonding patterns of guanine in duplex DNA. Kou, Y.; Koag, M.; Lee, S.* J. Am. Chem. Soc. 2015, 137, 14067.
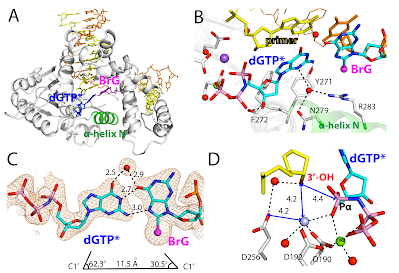
26. Structural basis for the inefficient nucleotide incorporation opposite cisplatin-DNA lesions by human DNA polymerase β. Koag, M.; Lai, L.; Lee, S.* J. Biol. Chem. 2014, 289, 31341.
25. The spontaneous replication error and the mismatch discrimination mechanisms of human DNA polymerase β. Koag, M.; Nam, K.*; Lee, S.* Nucleic Acids Res. 2014, 42, 11233.
24. Transition-state destabilization reveals how human DNA polymerase β proceeds across the chemically unstable lesion N7-methylguanine. Koag, M.; Kou, Y.; Ouzon, H.; Lee, S.* Nucleic Acids Res. 2014, 42, 8755-8766.
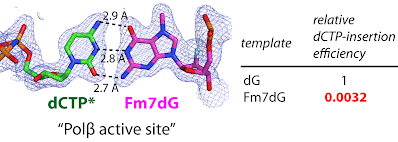
23. Streamlining bottom-up protein identification based on selective UVPD of chromophore-tagged histidine- and tyrosine-containing peptides. Aponte, J.; Vasicek, L.; Waminathan, J.; Xu, H.; Koag, M.; Lee, S.; Brodbelt, J. S.*Anal. Chem. 2014, 86, 6237-6244.
22. Metal-dependent conformational activation explains highly promutagenic replication across O6-methylguanine by human DNA polymerase β.Koag, M.; Lee, S.* J. Am. Chem. Soc. 2014, 136, 5709-5721. (Recommended for F1000Prime)
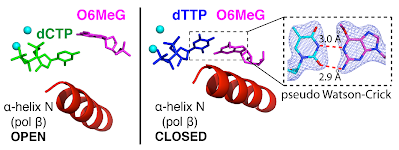
21. Structural basis for the promutagenicity of 8-bromoguanine. Koag, M.; Min, K.§; Lee, S. *J. Biol. Chem. 2014, 289, 6289-6298.
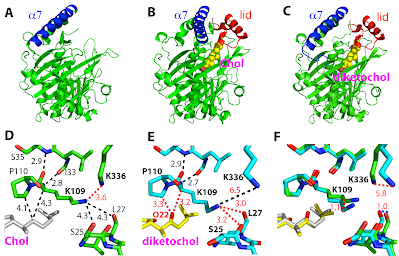
20. Synthesis and structure of 16,22-diketocholesterol bound to oxysterol-binding protein Osh4. Koag, M.; Cheun, Y.; Kou, Y.; Ouzon-Shubeita, H.; Min, K.§; Monzingo, A. F.; Lee, S.* Steroids, 2013, 79, 938-944.
19. Unexpected opening of steroidal E-ring during hypoiodite-mediated oxidation. Kou, Y.; Lee, S.*. Tetrahedron Lett. 2013, 54, 4106-4109.
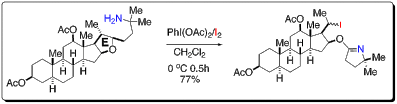
18. Synthesis of C17-OH-north unit of ritterazine G via “Red-Ox” modifications of hecogenin acetate. Cheun, Y.; Kou, Y.; Stevenson, B.; Kim, H.; Koag, M.; Lee, S.* Steroids, 2013, 78, 639-643.
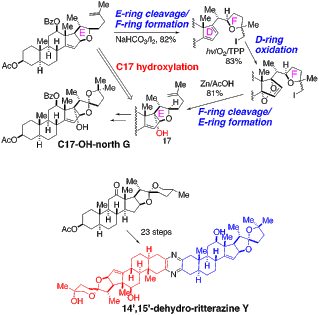
17. Synthesis of 14′,15′-dehydro-ritterazine Y via reductive and oxidative functionalizations of hecogenin acetate. Kou, Y.; Cheun, Y.; Koag, M.; Lee, S.* Steroids, 2013, 78, 304-311.
16. Application of hypoiodite-mediated aminyl radical cyclization to synthesis of solasodine acetate. Kou, Y.; Cheun, Y.; Koag, M.; Shin, A§.; Lee, S.* Steroids, 2012, 77, 1069-1074.
15. Transetherification-mediated E-ring opening and stereoselective “Red-Ox” modification of furostan.Cheun, Y.; Koag, M.; Kou, Y.; Warnken, Z.; Lee, S.* Steroids 2012, 77, 276-281.
14. Discovery of hypoiodite-mediated aminyl radical cyclization lacking a nitrogen radical-stabilizing group: Application to synthesis of an oxazaspiroketal-containing cephalostatin analog.Koag, M.; Lee, S.* Org. Lett., 2011, 13, 4766–4769.
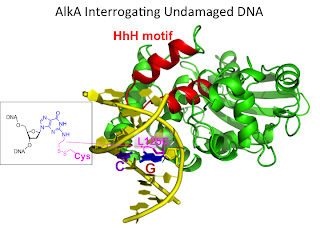
13. Structure of Escherichia coli 3-methyladenine DNA glycosylase AlkA bound to undamaged DNA. Bowman, B. R.; Lee, S.; Wang, S.; Verdine, G. L.* J. Biol. Chem. 2010, 285, 35783-791.
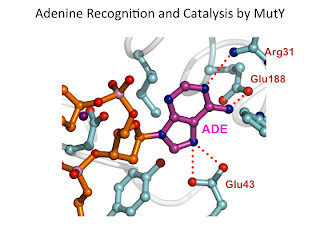
12. Atomic substitution reveals the structural basis for substrate adenine recognition and removal by adenine DNA glycosylase. Lee, S.; Verdine, G. L.* Proc. Natl. Acad. Sci. USA 2009, 106, 18497.
11. Chemistry of trisdecacyclic pyrazine antineoplastics: The cephalostatins and ritterazines. Lee, S.; LaCour, T. G.; Fuchs, P. L.* Chem. Rev. 2009, 109, 2275.
10. Synthesis of C14,15-dihydro-25-epi north unit of cephalostatin 1 via redox modifications of hecogenin acetate. Lee, S.*; Jamieson, D.; Fuchs, P. L.* Org. Lett. 2009, 11, 5.
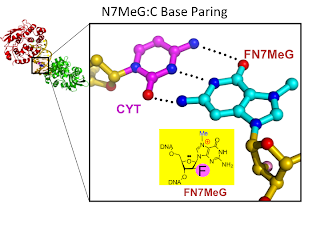
9. Synthesis and structure of duplex DNA containing the genotoxic nucleobase lesion N7-methylguanine. Lee, S.; Bowman, R. B.; Ueno, Y.; Verdine, G. L.* J. Am. Chem. Soc. 2008, 130, 11570.
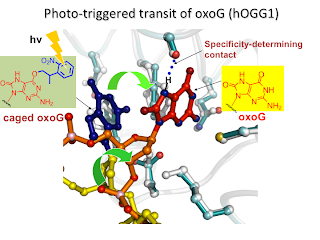
8. Trapping and structural elucidation of a very advanced intermediate in the lesion-extrusion pathway of hOGG1 (highlighted in Nat. Chem. Biol. 2008, 4, 395) Lee, S.; Radom, C. T. Verdine, G. L.* J. Am. Chem. Soc. 2008, 130, 7784.
7. Structure of the Escherichia coli DNA glycosylase AlkA bound to the ends of duplex DNA: A system for the facile structure determination of lesion-containing DNA.Bowman, B. R§.; Lee, S. §; Verdine, G. L.* Structure 2008, 16, 1166.
6. A Concise synthesis of 4′-F nucleic acids. Lee, S.; Utammapinat, C.; Verdine, G. L.* Org. Lett. 2007, 9, 5007.
5. In situ nitrosonium ion generation. Oximinylation of enol ethers from steroidal spiroketals. Introduction of C23-OH in cephalostatin intermediates. Lee, S.; Fuchs, P. L.* Can. J. Chem. 2006, 84, 1442.
4. An efficient C-H oxidation protocol for the α-hydroxylation of cyclic steroidal ethers. Highly efficient, regio- and stereoselective oxyfunctionalization of steroids by Cr[VI]. Lee, S.; Fuchs, P. L.* Org. Lett. 2004, 6, 1437.
3. Chemospecific chromium[VI] catalyzed oxidation of C-H bonds at -40 oC. Lee, S.; Fuchs, P. L.* J. Am. Chem. Soc. 2002, 124, 13978.
2. The first total synthesis of ritterazine M. Lee, S.; Fuchs, P. L.* Org. Lett. 2002, 4, 317..
1. Redox functionalization of steroid spiroketals. Structure correction of ritterazine M. Lee, S.; LaCour, T. G.; Lantrip, D. A.; Fuchs, P. L.* Org. Lett. 2002, 4, 313.
Principal Investigator:
Dr. Seongmin Lee
Associate Professor of Chemical Biology & Medicinal Chemistry (2015-present)
Assistant Professor: The University of Texas at Austin (2009-2015)
Research Associate: Harvard University (2007-2009)
Post-Doctoral Fellow: Harvard University (2004-2007)
Ph.D.: Purdue University
B.S.: Seoul National University, South Korea
Research Fellows:
Dr. Hunmin Jung (Ph.D. Texas A&M University, 2017-)
Graduate Students:
Aaron Rozelle: Chemical Engineering (B.S. North Carolina State University)
Kate Vilas: Biochemistry (B.S. The University of North Carolina at Chapel Hill)
Lillian Schmaltz: CMB (B.S. Northeastern University)
Nestor Rodriguez: CBMC (B.S. Texas State University)
Undergraduate Students:
Louis Zhang (Chemistry, April 2019-)
Laura Kim (Biology, April 2019-)
Samuel Lin (Biochemistry, April 2019-)
Andrew Ta (Chemistry, April 2019-)
Zachary Derrah (Chemical Engineering, January 2019-)
Former Group Members:
Dr. Naveen Kumar (Indian Institute of Technology, 2014-2019)
Jinqi Pei: Biochemistry (2016-2017)
Usha Thotakura: Biochemistry (2016-2017)
Suyang Li: Biochemistry (2016-2017)
Dr. Young Cheun: Medicinal Chemistry (Ph.D. The University of Texas at Austin)
Dr. Yi Kou: Biochemistry (Ph.D. The University of Texas at Austin)
Dr. Hala Ouzon-Shubeita (Ph.D. The University of Texas at Austin)
Dr. Myong-Chul Koag (Ph.D. UC Davis)
Dr. John Cheong (Ph.D. The University of Wisconsin at Madison)
Meghan Baker (2014-2016, Biochemistry)
Brant Campodonico (2014-2016, Biochemistry)
Erika Rodrigues (2015-2016, Plan II Honors)
Lara Lai (2013-2015, Nutritional Sciences, Pre-Pharmacy)
Dr. Heekwon Kim (2012-2103) Post-Doc, Current; Assistant Professor, Cheonbuk National University, South Korea
Dr. Byeongjae Lee (2009-2010) Post-Doc, Current; Korea Research Institute of Bioscience and Biotechnology
Kyungjin Min (2012-2013) Undergraduate Student
Courtney Coch (2012) Undergraduate Student
Aram Shin (2011-2012) Undergraduate Student, Current; School of Medicine, Ewah Womans University, South Korea
Jenny Choi (2010-2011) Undergraduate Student, Current; College of Pharmacy, The University of Houston
Jennifer Washburn (2009-2010) Undergraduate Student
Jeongeun Park (2009-2010) Undergraduate Student, Current; Massachusetts College of Pharmacy and Health Sciences
Yaejin Im (2009-2010) Undergraduate Student, Current; College of Pharmacy, The University of Houston
Jennifer Min (2013-2014) High school Student, Current; Emory University
Undergraduate and graduate students who are interested in studying DNA damage, replication, and mutagenesis and anticancer chemotherapeutics are encouraged to contact Professor Lee (seongminlee@austin.utexas.edu).
Email address:
Phone:
Campus location:
US Mail Address:
2409 University Avenue
The University of Texas at Austin
Austin, TX 78712

