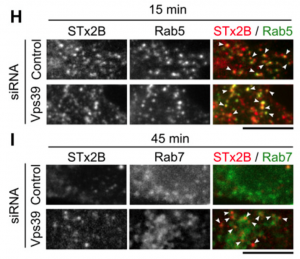
with control or Vps39 siRNA.
New research from a lab within the College of Pharmacy may have discovered a way to repurpose an existing drug to fight the lethality of Shiga toxin-producing Escherichia coli (STEC) infections. Somshuvra Mukhopadhyay, M.B.B.S., Ph.D., assistant professor in the Division of Pharmacology and Toxicology, recently led research focused on stemming the spread of Shiga toxins released by E. coli bacteria and potentially fatal if they reach the kidneys. Currently, there are no treatments for STEC infections. Antibiotic therapy cannot be used because it increases release of Shiga toxins from the bacteria, and approved antidotes are not available to neutralize the effects of these toxins.
Dr. Mukhopadhyay’s research initially focused on understanding the means by which the Shiga toxins enter human cells and cause cell death. The research group discovered that the acidity of certain intracellular compartments played a critical role in allowing the toxins to successfully invade cells. The group then screened for drugs that may alter cellular acidity as a way to protect against the toxins. These studies led to the discovery that tamoxifen, an FDA approved drug for treatment of other diseases such as breast cancer, potently blocks the capability of the Shiga toxins to invade human cells and cause disease. Subsequent studies revealed that tamoxifen also protects mice against lethal Shiga toxicosis. If further research supports tamoxifen’s effectiveness in managing the spread of Shiga toxins, it may pave the way for treatment of humans with deadly E. coli. It may also be possible to combine tamoxifen with antibiotics to rapidly clear patients of STEC bacteria.
One significant advantage of these findings is, since tamoxifen is an existing drug deemed safe by the FDA, its use as a treatment to fight E. coli may reach patients earlier than a brand-new medication. “The ability to repurpose an old drug to treat Shiga toxicosis is exciting because it may be possible to advance this mode of therapy into clinical practice more rapidly than a new compound,” says Dr. Mukhopadhyay.

The research findings already have one interested party seeking to capitalize on the solution. “We hope to license Dr. Mukhopadhyay’s discovery and make it available to the public; in the past year alone, the United States has experienced three major outbreaks of foodborne Shiga toxin-producing E. coli spanning 40 states,” said Kristin Falkenstein, Ph.D., licensing specialist at the UT Austin Office of Technology Commercialization. “It offers a promising therapeutic solution using an FDA approved drug in the face of an increasingly prevalent epidemic.” Housed in UT Austin’s Office of the Vice President for Research, the Office of Technology Commercialization is responsible for the efficient transfer of university discoveries to the marketplace for the benefit of society.
Infections by Shiga toxin-producing E. coli are a major cause of lethal food-borne disease in the United States. According to the Centers for Disease Control and Prevention (CDC), an estimated 265,000 people in the United States are affected by STEC each year, resulting in approximately 3,600 hospitalizations and 30 deaths. STEC O157, the most lethal E. coli strain, causes 36 percent of those infections. In approximately 5-15 percent of patients with STEC O157 infections, the Shiga toxins released by the bacteria reach the kidneys where they kill kidney cells, potentially resulting in hemolytic uremic syndrome (HUS), a type of kidney failure. Many people require dialysis. Most people with this condition recover within a few weeks, but some suffer permanent kidney damage or die. Young children and older adults are more likely to develop HUS.
Dr. Mukhopadhyay’s research article, titled “Tamoxifen blocks retrograde trafficking of Shiga toxin 1 and 2 and protects against lethal toxicosis,” was recently published by Life Science Alliance. Other researchers include Andrey S. Selyunin, Ph.D. a post-doctoral research fellow in the College of Pharmacy’s Division of Pharmacology and Toxicology, Steven Hutchens, B.S., research associate and lab manager in the Mukhopadhyay Lab, and Stanton F. McHardy, Ph.D., director of The University of Texas at San Antonio’s Center for Innovation in Drug Discovery. This work was funded by a grant from the National Institute of Allergy and Infectious Diseases (NIAID).
The Mukhopadhyay Lab in The University of Texas at Austin College of Pharmacy focuses on understanding cell biology of human disease. In addition to its work on intracellular trafficking of Shiga and related bacterial toxins, the lab also conducts research projects involving parkinsonism and metal homeostasis. Dr. Mukhopadhyay’s research on manganese regulation was recently highlighted as one of the National Institute of Environmental Health Sciences (NIEHS) Papers of the Month.
Download the news release about this important discovery.

