TTP includes a multi-disciplinary team of scientists with expertise in specialized research technologies, such as assay development, laboratory automation, high-throughput chemical screening, chemoinformatic analysis, and molecular & cell biology essential for cutting-edge, targeted drug discovery research to maintain the resources and perform the services outlined below. Each service can be offered as a standalone or embedded in a total discovery pipeline.
Please review their respective sections below for additional details.
- Assay Development and Small Molecule Screening
- Compound Management
- Medicinal Chemistry
- Research Informatics
- Lead Characterization
Drug Discovery Pipeline
We will consider a project to be successful if it results in the discovery of one or more potent small molecules that possesses PK parameters as follows: good oral bioavailability (>30 %F), half-life of >3 hours, plasma exposure (2000 hr*ng/mL, AUClast).
We envisage TTP being the only program in our region of Texas dedicated to offering an extensive and project-customized drug discovery pipeline (see figure below) for targeted-therapeutics, which ultimately will furnish compounds exhibiting pharmacodynamics parameters that enable complete inhibition of target function for 24 hours in plasma and bone marrow following once or twice daily oral doses of 50 mg/Kg or less.
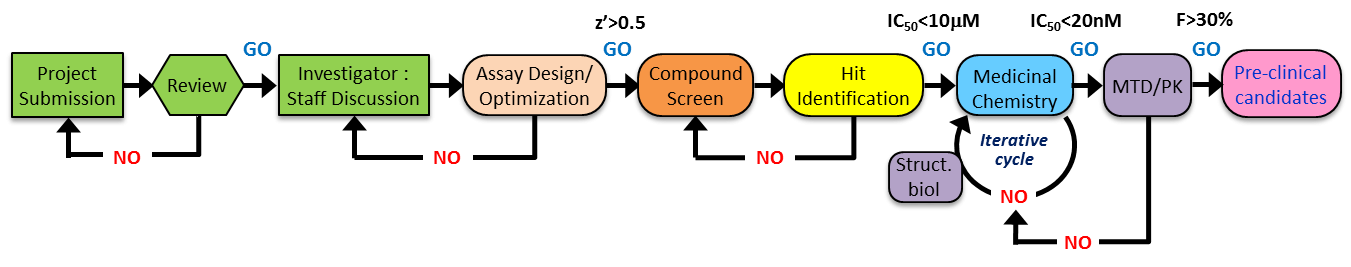
What We Offer
Assay Development and Small Molecule Screening
TTP has strong expertise in “Hit Identification” utilizing diverse screening platforms (biochemical, cellular, virtual, fragment-focused, and phenotypic).
- Support for target-based assay design and optimization, applicable for robust and sensitive compound screening, hit validation, and lead optimization.
- Support flexible and custom-tailored screening strategies
- Support high-quality recombinant protein/enzyme expression and purification
- Execution of small molecule screens and validation of hits
- Support for screening data management (analysis and archive screening data) and reporting of analyzed data to investigators
- Provision of advice and/or services regarding the delineation of the modality of inhibition by hit compounds
TTP offers a wide variety of detection modalities and formats for assay measurements that include:
- Absorbance, Fluorescence, TRF, FRET, FP, Luminescence, Alphascreen, Circular Dichroism, DSF, SPR
- Cellular imaging (DAPI, GFP, RFP, CFP, YFP; 4x, 10x, 20x, 40x)
- Homogeneous (i.e., “mix and read”) and other types of heterogeneous assays (ELISA)
- PPI (protein:protein interaction), reporter assay, enzymatic activity assay, cell counting, viability, proliferation and cytotoxicity.
TTP’s state-of-the-art instrumentation provides a highly flexible screening campaign at various throughputs
- Spectra and well area scan
- End-point or kinetic measurement
- 96-, 384- and 1,536-well plate formats
- > 50,000 compounds per experiment
Compound Management
- Identify and curate novel compounds into the chemical library from commercial sources and collaborating laboratories
- Distribute subset of compound collections or cherry pick libraries.
- Assist compound procumbent
- Manage chemical and biological database of all registered small molecules
Medicinal Chemistry
TTP supports chemistry projects at any phase of a drug discovery program (hit validation, expansion, hi-to-lead development, and lead optimization) across a wide range of compound types, target classes, disease areas. TTP’s chemists have expertise in both protein structure-guided and ligand-based design utilizing both experimental knowledge and computational modeling.
- Provision of advice and/or service regarding structure-guided synthesis of new analogs
- Design and synthesis of hit or lead analogs
- Scale up synthesis for lead progression
- Develop strategy for and securing IP protection
Research Informatics
TTP offers an integrated discipline of computational chemistry, bioinformatic system, and data management to support:
- Development of preliminary Structure Activity Relationships (SAR) for hit compounds.
- Identification of structurally similar commercially available analogs via structure-based docking or pharmacophore searching (hit expansion)
- In silicon design
- Virtual screen
Computing Facility
- Computing Cluster (Ren Lab, 500-core Dell Linux cluster with Gigabit network and a 16 TB disk storage): Virtual screening, drug library design, database searching and data file storage.
- GPU Computing Cluster (Ren Lab, 12 GTX1070 GPUs): Molecular dynamics simulations; lead optimization.
- High performance computing cluster (Lonestar 5, access through TACC, 30048 compute cores, 5 PB disk storage, 40 GPUs): Molecular modeling and molecular dynamics simulations of protein-ligand binding.
- Workstations (Tx-Sact, High-end Dell graphics workstation): Visualization and manipulation of molecular systems. Job benchmarking and submission.
Software Capability
- De novo ligand library design (Daylight Reaction Toolkit): De novo ligand design by linking reactive agents to fragment scaffold.
- Ligand based screening (ROCS and EON, OpenEye): Shape and electrostatic similarity based ligand search.
- Virtual screening (GOLD (CCDC)): Screening of small molecule library against protein targets, predicting binding poses and affinity ranking.
- Molecular Modeling (AMBER, GROAMCS, TINKER, OpenMM): Molecular dynamics simulations of protein-ligand binding; advanced binding affinity/free energy calculation for ligand screening and optimization.
- Visualization (Datawarrior, Pymol, VMD, Chimera): Chemical space visualization and manipulation of protein-ligand systems.
Lead Characterization
Identification of “Lead candidate” is another critical step in the drug discovery pipeline. For this challenging phase, TTP scientists work closely with investigators as well as core facilities on campus and GCC member institutes to achieve efficient Lead Characterization.
- Support target engagement using various in biophysical techniques (DSF, CD, SPR, BRET, NMR*, MS*)
- Support mechanism of action (MOA) studies
- Provide advice and/or service regarding the formulation* and evaluation of in vivo compound bioavailability* (pharmacokinetics studies) to facilitate in vivo efficacy studies
* Supports in integration with shared facilities.
Resources - Drug Screening SOP
Early Stage Drug Discovery Guidance
Assay Guidance Manual: A free, best-practices online resource devoted to the successful development of robust, early-stage drug discovery assays. The manual was originally developed by Eli Lilly and Company to provide step-by-step guidance based on “tribal knowledge” from drug developers for planning and creating projects for high-throughput screening, lead optimization and early phases of regulated drug development. A series of brief audio presentations can be found in a two-day workshop.
Cambridge MedChem Consulting: A resource provided by a commercial sector for scientists undertaking drug discovery, categorized by Target Validation, Hit Identification, Lead Identification, Lead Optimization, ADME Properties, Pre-Clinical Toxicity, Miscellaneous, and
Preclinical Checklist.
Reporting data from high-throughput screening of small-molecule libraries
Biophysics in drug discovery
Statistical practice in high-throughput screening
SLAS Discovery Special Issue (Volume 26 Issue 10, December 2021): Assay Guidance Manual for Drug Discovery: Robust or Go Bust
For additional information and to submit a screening application, please reach out to our office.

